|
SODIUM HEXAMETAPHOSPHATE
| ||
PRODUCT IDENTIFICATION | ||
| CAS NO. | 10124-56-8 |
|
| EINECS NO. | 233-343-1 | |
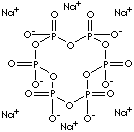
| FORMULA | (NaPO3)6 | |
| MOL WT. | 611.77 | |
H.S. CODE |
2835.39.5000 | |
| TOXICITY | Oral rat LD50: 6200 mg/kg | |
| SYNONYMS | Metaphosphoric acid, hexasodium salt; Calgon S; | |
| SHMP; Glassy sodium; Hexasodium metaphosphate; Metaphosphoric acid, hexasodium salt; Sodium Polymetaphosphate; sodium polymetaphosphate; Graham's Salt; Graham's salt; SHMP; Other RN: 8012-14-4, 16210-45-0, 20517-55-9, 193678-44-3 | ||
| SMILES | P1(OP(OP(OP(=O)([O-])OP(OP(O1)(=O)[O-])(=O)[O-])(=O)[O-] )(=O)[O-]) (=O)[O-].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+] | |
CLASSIFICATION |
Phosphate | |
EXTRA NOTES |
| |
PHYSICAL AND CHEMICAL PROPERTIES | ||
| PHYSICAL STATE | White powder | |
| MELTING POINT |
| |
| BOILING POINT | ||
| SPECIFIC GRAVITY | 2.18 | |
| SOLUBILITY IN WATER | Soluble | |
| pH | 5.5 - 7.7 | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
| |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION | ||
Wikipedia Linking - Sodium hexametaphosphate Google Scholar Search - Sodium hexametaphosphate Drug Information Portal (U.S. National Library of Medicine) - Sodium hexametaphosphate PubChem Compound Summary - Sodium hexametaphosphate IPCS INCHEM - PHOSPHORIC ACID AND PHOSPHATE SALTS KEGG (Kyoto Encyclopedia of Genes and Genomes) - Sodium hexametaphosphate http://www.ebi.ac.uk/ - Sodium hexametaphosphate http://www.ncbi.nlm.nih.gov/ - Sodium hexametaphosphate http://toxnet.nlm.nih.gov/ http://www.phosphatesfacts.org/ Local: | ||
| SALES SPECIFICATION |
TECH GRADE |
FOOD GRADE |
|
APPEARANCE |
White Powder | |
| ACTIVE MATTER |
68.0% min |
68.0% min |
| INSOLUBLES IN WATER |
0.05% max |
0.05% max |
|
pH |
5.8 - 7.3 |
5.8 - 6.5 |
| Fe |
0.005% max |
0.005% max |
| As |
|
3ppm max |
| HEAVY METALS |
|
10ppm max |
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
|
SAFETY INFORMATION |
||